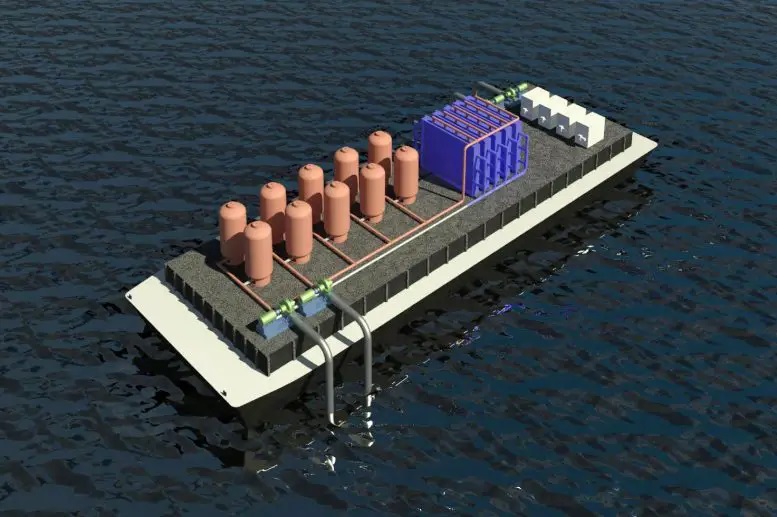
A new technique has been discovered by researchers to be effective in removing carbon dioxide from the ocean. It could be utilized by ships that process seawater while traveling, or at offshore drilling platforms or aquaculture fish farms.
Researchers have developed a new method for removing greenhouse gas from the ocean, which could be much more efficient than existing systems for removing it from the air. As carbon dioxide continues to accumulate in the Earth’s atmosphere, research teams worldwide have been searching for years to find efficient ways to remove the gas from the air.
The ocean is the world’s top “sink” for carbon dioxide in the atmosphere which soaks around 30-40 percent of the gas produced by human activities. The direct extraction of carbon dioxide from ocean water has become a promising option for mitigating CO2 emissions, potentially leading to the reduction of overall negative emissions.
According to a team of researchers at MIT, they may have discovered the key to a highly efficient and cost-effective removal mechanism. In a paper published in the journal Energy and Environmental Science, professors from MIT and Harvard University recently reported on the findings.
The current methods for removing carbon dioxide from seawater use a voltage across a stack of membranes to acidify a feed stream by water splitting. By doing this, bicarbonates in water are converted into molecules of CO2, which can be excreted under vacuum.
The cost of the membranes and the need for chemicals to drive the overall electrode reactions at either end of the stack add to the expense and complexity of the processes, according to Hatton, who is the Ralph Landau Professor of Chemical Engineering. According to him, the aim was to prevent the introduction of chemicals into the anode and cathode half cells and to avoid the use of membranes if possible.
At the outset, the system can utilize existing or planned infrastructure that already processes seawater, such as desalination plants, but the system is scalable.
By utilizing reactive electrodes, protons are released into the seawater that is fed to the cells, leading to the release of dissolved carbon dioxide from the water. The process is cyclic and involves acidification of water to convert dissolved inorganic bicarbonates into molecular carbon dioxide, which is then collected as a gas under vacuum. The water is then transferred to a second set of cells with a reversed voltage, which retrieves the protons and restores the acidic water to its alkaline state before returning it to the sea. The functions of the two cells are periodically reversed when one set of electrodes is depleted of protons (during acidification) and the other has been regenerated during alkalization.
As with other carbon removal processes, it is still necessary to dispose of the carbon dioxide that has been removed from the water. It can be buried in deep geologic formations beneath the sea floor, or chemically transformed into a compound like ethanol, which can be used as a transportation fuel, or into other specialty chemicals. It can be buried in deep geologic formations beneath the sea floor, or chemically converted into a compound like ethanol, which can be used as a transportation fuel, or into other specialty chemicals.
The research is ongoing, with the aim of finding a substitute for the current method that necessitates a vacuum to remove the separated carbon dioxide from the water. There is a need to identify operating methods that prevent the precipitation of minerals that can foul the electrodes in the alkalinization cell, an inherent problem that reduces the overall efficiency in all reported approaches.
Within the next two years, the team hopes to have the system ready for a practical demonstration project.
Reference: “Asymmetric chloride-mediated electrochemical process for CO2 removal from oceanwater” by Seoni Kim, Michael Nitzsche, Simon B Rufer, Jack R. Lake, Kripa Kiran Varanasi and T. Alan Hatton, 13 February 2023, Energy & Environmental Science.